Science: Section 3
Combustion of Fossil Fuels
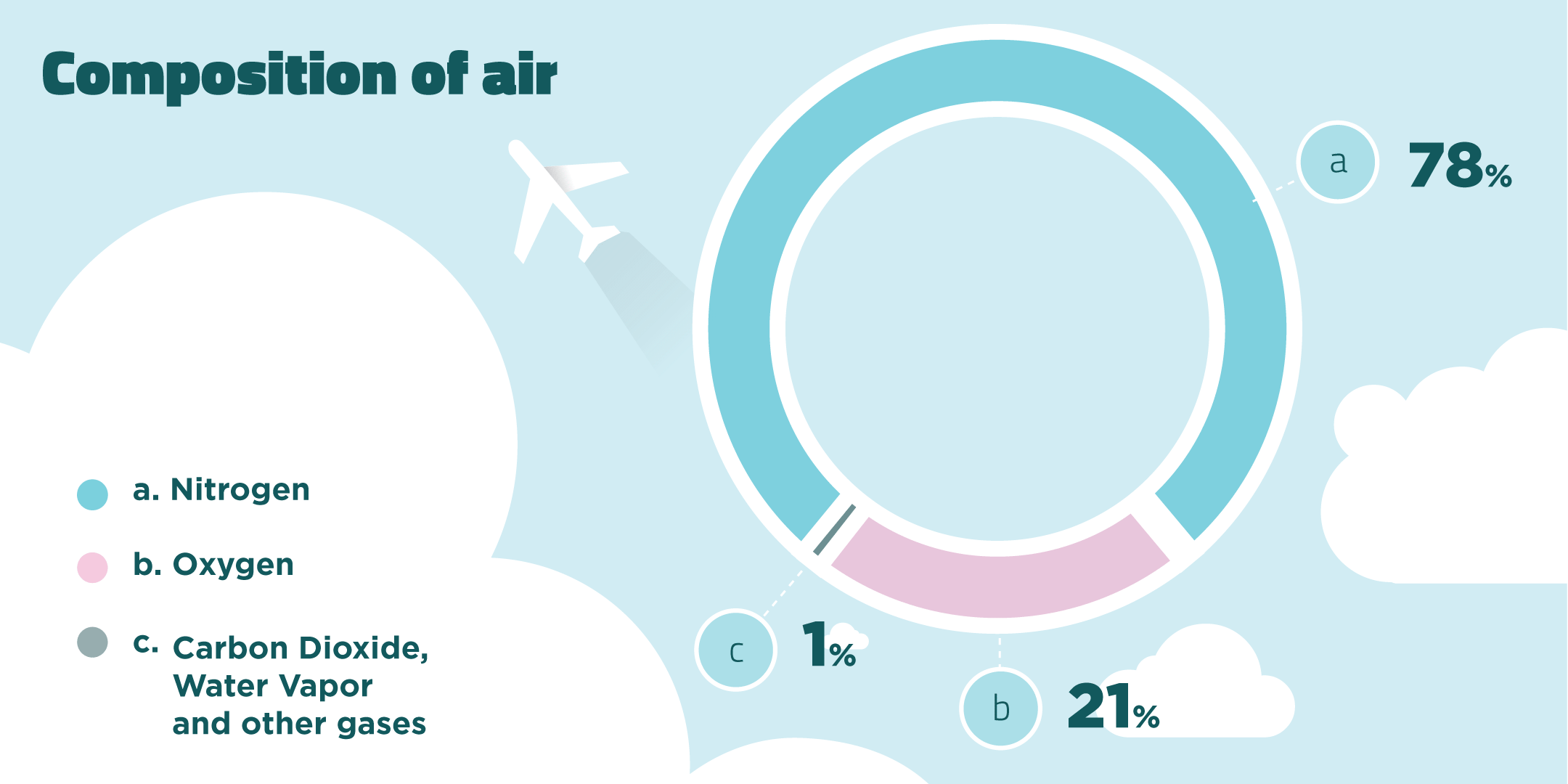
When fuels burn they react with oxygen in the air. Air is a mixture of gases made up of mainly nitrogen and oxygen gas.
Products of Combustion
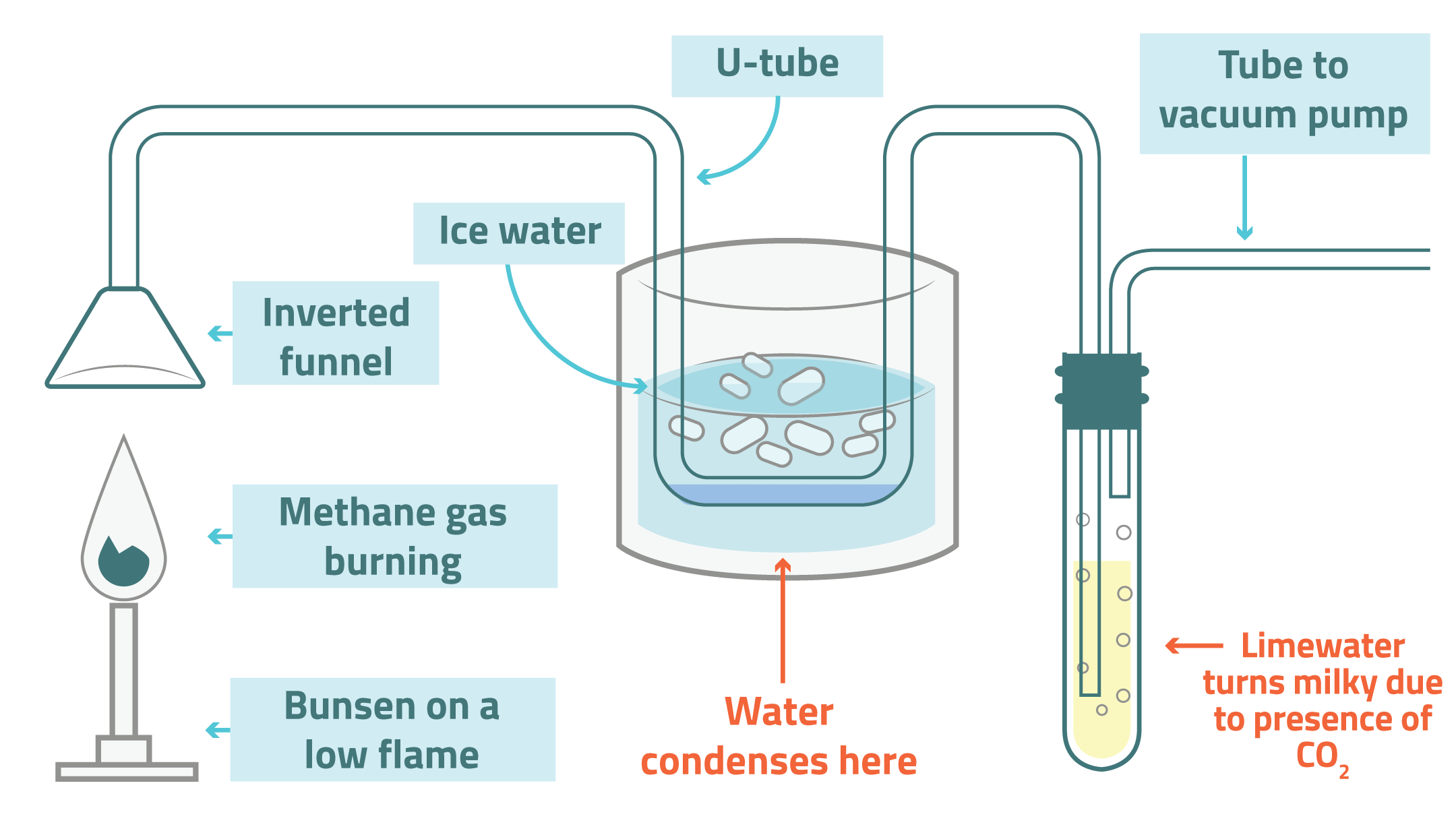
When a hydrocarbon burns in a plentiful supply of oxygen, carbon dioxide and water are produced.

This can be shown by the following experiment:
Click here to show the Products of Combustion Experiment Video
Instruct the pupils to construct a table of observations that can be made as the reaction proceeds and record colour changes including the soot produced that collects on the funnel.
Other products of combustion …
When a hydrocarbon burns in a limited supply of oxygen, carbon monoxide and fine particles can be produced which can affect health.

Carbon monoxide - a highly poisonous gas that is both colourless and odourless (nicknamed the silent killer!)
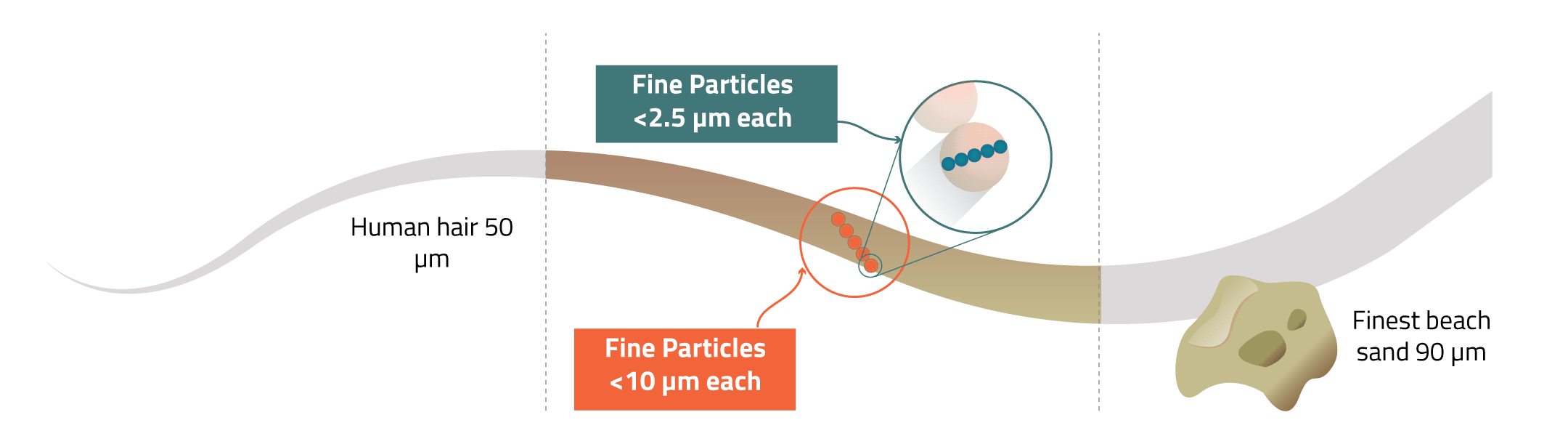
Fine Particles - tiny pieces of solid matter or liquids in the air that are released during combustion. Some are large enough to be seen with the naked eye, such as black soot, however others can only be seen through powerful microscopes. The smaller particles can be breathed deep into your lungs and then even go into your bloodstream causing health problems.
When a hydrocarbon burns at high temperatures in the air nitrogen dioxide can be produced.
Nitrogen dioxide is a poisonous reddish, brown gas with an unpleasant smell. Most of the nitrogen dioxide in urban areas comes from exhaust emissions, but it can also be formed naturally by lightning strikes.
Click here to show the Sparking Air Demonstration Video
Sulfur dioxide is a colourless gas that is formed when fuels containing sulfur, such as coal and oil, are burned. It is also produced from natural sources such as active volcanoes and hot springs. Sulfur dioxide is toxic to plants and can cause breathing difficulties and acid rain.
Click here to show the Burning Sulphur Demonstration Video
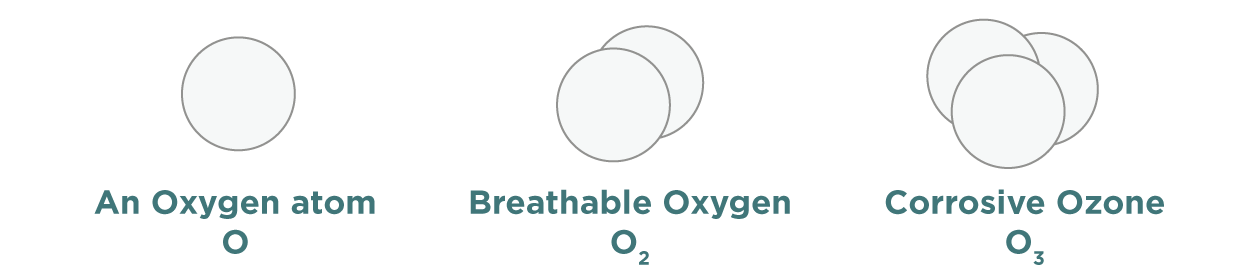
Ozone (O3) is a colourless gas made up of oxygen. It is formed by hydrocarbons and nitrogen dioxide reacting in the presence of sunlight. Ozone is highly reactive, making it useful for cleaning and disinfecting. But, when it comes in contact with living tissues like our lungs it can cause damage and illness. Ozone can also damage plants, reducing the growth of important food crops such as wheat.
There is also a Homework Sheet you can download that supports and develops further the ideas from this section.
